Datatrak Enterprise Cloud®
The cost-effective solution embedded with full functionality
Approximate 40-50 new drugs are approved on a year basis. The new development starts with searching for candidate substances and continues with various clinical trials. On average, it takes at least ten years for a new medicine and costs enormously to complete the journey from initial discovery to the marketplace.
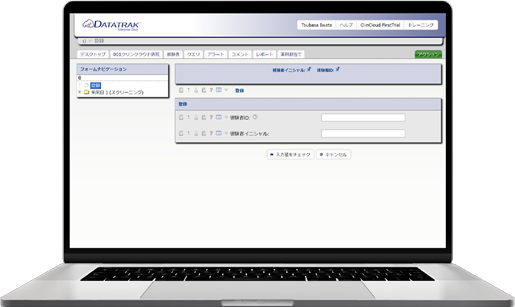
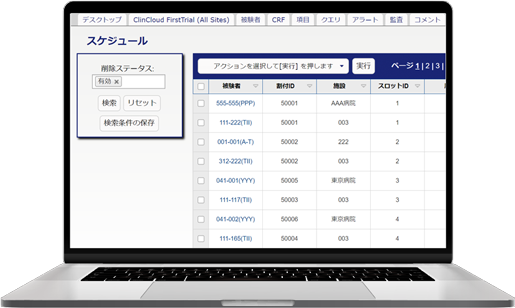
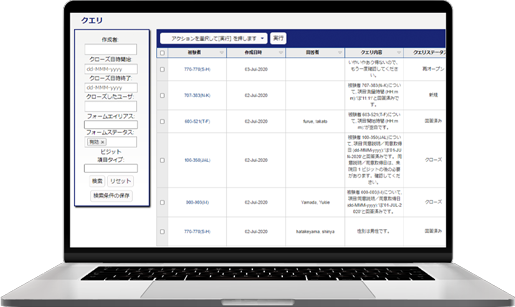
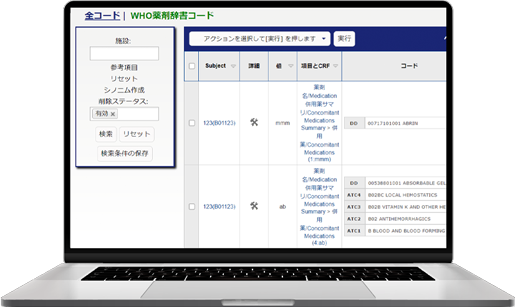
We provide our EDC (Electronic Data Capture) for clinical studies, Fountayn(Pre Datatrak Enterprise Cloud). In the Electric Data Capture, all over the world 83 counties, more than 10,000 studies have been run and it is the most proven track record of EDC system. All the features required of EDC system are aggregated and included in one platform and user interface. Experience our services with Datatrak Enterprise Cloud to reduce costs and the efficiency in conducting clinical studies.